The National Agency for Food and Drug Administration and Control (NAFDAC) has issued a public alert over falsified versions of two essential medicines, warning of serious health risks.
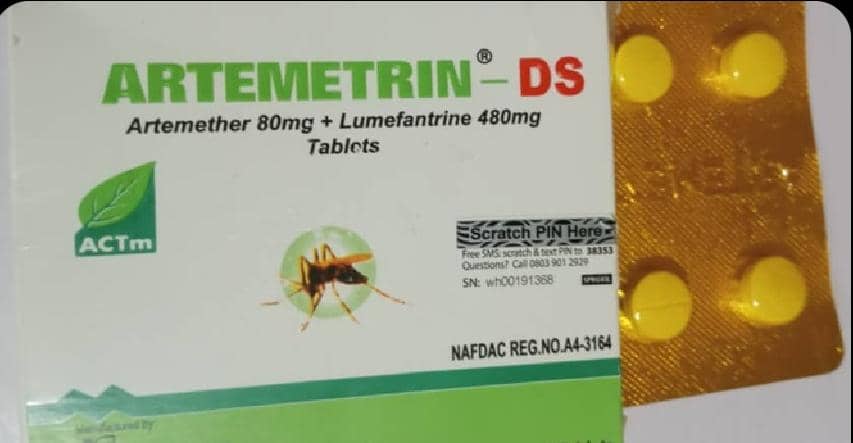
In a statement numbered 030/2025, the agency said counterfeit batches of the anti-malarial Artemetrin DS and the antibiotic Ciprofit 500 were in circulation, despite being sourced from licensed vendors and wholesalers.
Laboratory tests showed the products contained dangerously low levels of active ingredients. NAFDAC said Artemetrin DS, labelled as manufactured by A.C. Drugs Ltd in Enugu State, contained only 59.2 per cent artemether and 71.2 per cent lumefantrine — well below the 90-110 per cent potency limit.
Ciprofit 500, falsely labelled as produced by Impact Pharmaceutical Ltd, was found to contain just 5.7 per cent ciprofloxacin. Neither drug is registered in NAFDAC’s official database and the registration numbers printed on the packaging were fabricated.
“The implications of substandard anti-malarials in a malaria-endemic country like Nigeria cannot be overstated,” a NAFDAC spokesperson said in the alert. “These falsified products undermine treatment efficacy and could lead to preventable deaths.”
The agency urged the public to stop using the medicines and return the stocks to its nearest office. Those who have taken the drugs and experienced adverse reactions or treatment failure should seek immediate medical attention.
The World Health Organisation estimates that substandard and falsified medicines make up about 10 per cent of the global market. In Nigeria, prevalence may be higher, complicating the fight against malaria, which kills over 200,000 people annually, and bacterial infections, which contribute to high morbidity.
The latest warning comes after NAFDAC seized fake malaria drugs worth 1.2 billion naira ($750,000) in Lagos on 12 September. Earlier alerts this year covered falsified Postinor-2 contraceptives, Oxytocin injections, and milk products. The regulator has pledged to reduce falsified medicine prevalence to below five per cent by the end of 2025.
NAFDAC advised healthcare providers, distributors, and consumers to verify products using its online database, purchase only from authorised outlets, and report suspicious cases via its hotline or dedicated email.
The agency’s director-general, Prof. Mojisola Adeyeye, reaffirmed its commitment to tackling counterfeit medicines. “With the full support of the government, we will continue to intensify surveillance and enforcement to eliminate these threats,” she said in a recent address.
WARNING: If You Are Not 18+, Don’t Click The Link Below 👇🫣
https://facultativethus.com/kx6iepv2qm?key=6c14bd1d68e1eba721851f19778f5efe
Please don’t forget to “Allow the notification” so you will be the first to get our gist when we publish it.
Drop your comment in the section below, and don’t forget to share the post.
Never Miss A Single News Or Gist, Kindly Join Us On WhatsApp Channel:
https://whatsapp.com/channel/0029Vad8g81Eawdsio6INn3B
Telegram Channel:
https://t.me/gistsmateNG